Abstract:
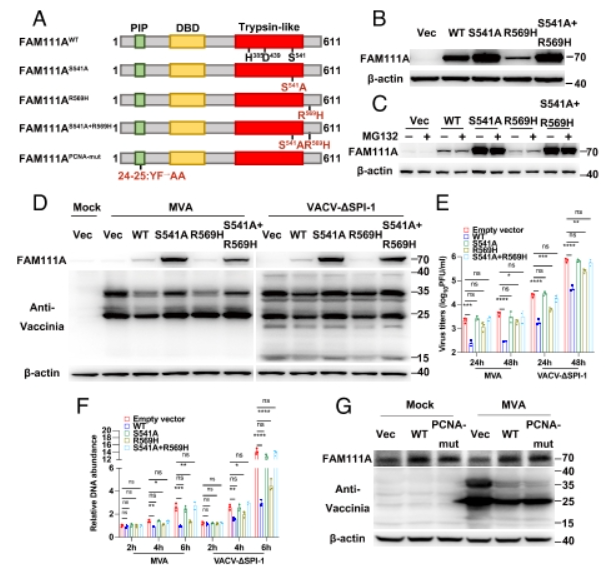
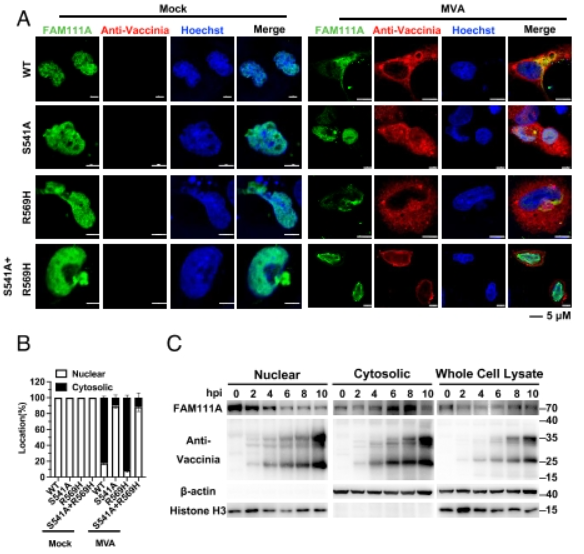
Zoonotic poxviruses such as mpox virus (MPXV) continue to threaten public healthsafety since the eradication of smallpox. Vaccinia virus (VACV), the prototypic poxvirusused as the vaccine strain for smallpox eradication, is the best-characterized member ofthe poxvirus family. VACV encodes a serine protease inhibitor 1 (SPI-l) conserved in allorthopoxviruses, which has been recognized as a host range factor for modified VACVAnkara (MVA), an approved smallpox vaccine and a promising vaccine vector, FAM111A(family with sequence similarity lll member A), a nuclear protein that regulates hostDNA replication, was shown to restrict the replication ofa VACV SPI-I deletion mutant(VACV-ASPI-1) in human cells. Nevertheless, the detailed antiviral mechanisms ofFAM111A were unresolved. Here, we show that FAM111A is a potent restriction factorfor VACV-ASPI-1 and MVA. Deletion of FAM111A rescued the replication of MVAand VACV-ASPI-1 and overexpression of FAM111A significantly reduced viral DNAreplication and virus titers but did not affect viral early gene expression. The antiviraleffect of FAM111A necessitated its trypsin-like protease domain and DNA-bindingdomain but not the PCNA-interacting motif. We further identified that FAM111Atranslocated into the cytoplasm upon VACV infection by degrading the nuclear porecomplex via its protease activity, interacted with VACV DNA-binding protein I3, andpromoted I3 degradation through autophagy. Moreover, SPI-1 from VACV MPXV orlumpy skin disease virus was able to antagonize FAM111A by prohibiting its nuclearexport. Our findings reveal the detailed mechanism by which FAM111A inhibits VACVand provide explanations for the immune evasive function ofVACV SPI-l.
Key Words:
poxvirus modified vaccinia virus Ankara (MVA) antiviral responsevirus-host interaction