Abstract:
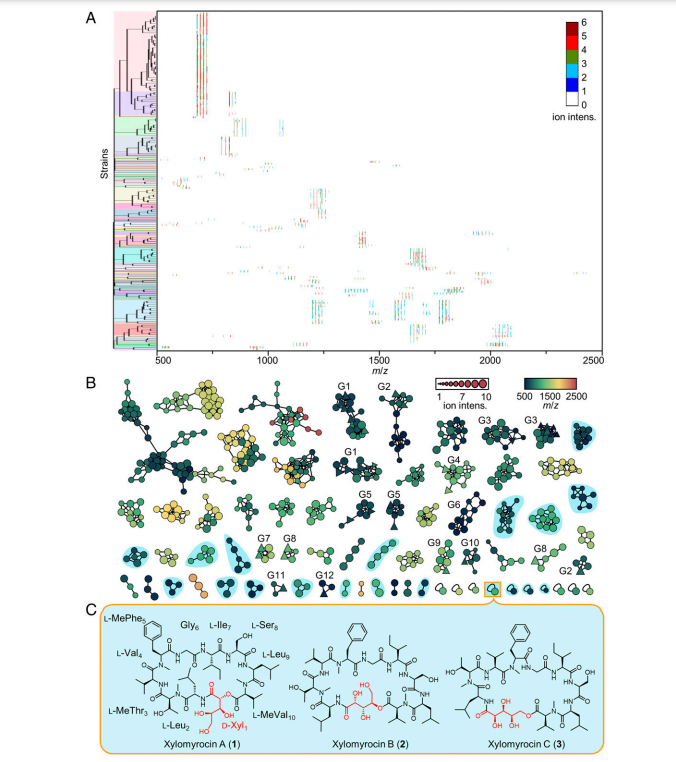
Xylomyrocins, a unique group of nonribosomal peptide secondary metabolites, were discovered in Paramyrothecium and Colletotrichum spp. fungi by employing a combination of high-resolution tandem mass spectrometry (HRMS/MS)–based chemometrics, comparative genome mining, gene disruption, stable isotope feeding, and chemical complementation techniques. These polyol cyclodepsipeptides all feature an unprecedented D-xylonic acid moiety as part of their macrocyclic scaffold. This biosynthon is derived from D-xylose supplied by xylooligosaccharide catabolic enzymes encoded in the xylomyrocin biosynthetic gene cluster, revealing a novel link between carbohydrate catabolism and nonribosomal peptide biosynthesis. Xylomyrocins from different fungal isolates differ in the number and nature of their amino acid building blocks that are nevertheless incorporated by orthologous nonribosomal peptide synthetase (NRPS) enzymes. Another source of structural diversity is the variable choice of the nucleophilefor intramolecular macrocyclic ester formation during xylomyrocin chain termination. This nucleophile is selected from the multiple available alcohol functionalities of the polyol moiety, revealing a surprising polyspecificity for the NRPS terminal condensation domain. Some xylomyrocin congeners also feature N-methylated amino acid residues in positions where the corresponding NRPS modules lack N-methyltransferase (M) domains, providing a rare example of promiscuous methylation in the context of an NRPS with an otherwise canonical, collinear biosynthetic program.
Keywords:fungal nonribosomal peptides;;xylonic acid ;;cyclodepsipeptides;; molecular networking ;; natural product dereplication